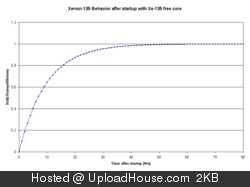
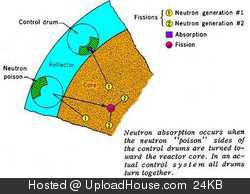
Xe-135 has such a large neutron cross-section that the vast majority of Xenon-135 atoms will never decay in a reactor – they absorb a neutron and become stable Xe-136 before they get a chance to decay.
One can think of Xe-135 as being equivalent to negative one neutrons, because each atom of Xe-135 is likely to absorb a neutron. It’s fission yield is about 6.3% So one way of looking at it is that at equilibrium operation, 6.3% of fission reactions will result in one less neutron.
It’s a fairly large hit on neutron economy, but not a deal-breaker. You can still have a reactor with plenty of neutron economy even with Xe-135. The place that it makes the big difference is stability. The fact that Xenon-135 decays and that it absorbs neutrons as it is irradiated means that when a reactor is shut down or brought to low power, it can be very unstable and the characteristics change as Xe-135 decays. For this reason, when a reactor is shut down it may be necessary to wait for the Xe-135 to decay away before restarting.
There is a cost to removing this stuff. If you leave it in, the vast majority will absorb a neutron and become Xe-136, which is not really a concern of any kind. If removed it will instead decay and it decays to Cs-135.
Removing the Xenon-135 can just about double the yield of Cs-135 from a reactor. Many find that very objectionable because Cs-135 is a long lived radioactive nucleotide.
But Cs-135 is only mildly radioactive, going through a single beta decay to Ba-135 with a half-life of 2.6 million years. Yes some people will worry about it but people will worry about all manner of things that aren’t worth worrying about. I think this is one of those cases.
Back-of-envelope estimate for the time available to extract the Sm-149, for anyone else thinking about this:-
A 1000 MW thermal neutron LFTR power plant requires an inventory of about 1000 kg of U-233 to run, and ‘burns’ about the same quantity per year. An individual U-233 atom in such a reactor therefore has a chance of about 1/365 per day of being hit by a neutron and fissioned.
The thermal neutron fission cross section of U-233 is 530 barns, the absorption cross-section of Sm-149 is about 40,000 barns, about 75 times greater (figures are from JENDL). Therefore the chance of a Sm-149 atom in the above reactor absorbing a neutron is about 75/365 per day or about 1/5. So to halve the loss of neutrons to samarium, you have got to process all the core salt – probably about 20 cubic metres – in five days. Around 170 litres/hr, just under 2 oz/sec
It is suppose it would be valuable to take all the lanthanides out, if such a thing could be done relatively simply. Way back when, ORNL looked at overloading the salt with cerium trifluoride, then cooling the salt and causing the lanthanides to preferentially freeze first, and skim them off. Cerium has a much smaller neutron absorption cross section than the other lanthanides like samarium, europium, and neodymium.
I never really liked that approach much because it seemed like you were cramming the salt full of something you’d rather not anyway. If an electrolytic cell could remove lanthanides online, all the better.
Many of the lanthanides reach nuclear stability quite quickly, but they’re still significant neutron poisons. Samarium-149 is the best example of this.
p/s: in the class, we will cover the Xe-135 and Cs-135

0 comments:
Post a Comment